We have seen a lot of research related to quantum computing and quantum communications . With a quantum computer it is possible to process information at unthinkable speeds, ideal for mathematical calculations. In communication, we have forms of transmission that are impossible to break. But, why have you never heard of quantum batteries ?
It is normal that you have not heard it, since this is the first time that a group of scientists has demonstrated the application of the superabsorption principle of quantum mechanics in a real device. Quantum physics is full of phenomena and concepts that may seem impossible, and superabsorption is one of them.

Super absorption to create quantum batteries
In superabsorption , molecules can become so entangled with each other that they can begin to act collectively , in this case increasing a molecule’s ability to absorb light. Specifically, this collective effect is that the transitions between the states of the molecules constructively interfere with each other. This type of interference occurs in all types of waves, including light, sound or water, and occurs when different waves add up to produce a greater effect than they would separately.
Thus, instead of generating a larger wave in the water, this effect allows the combined light molecules to absorb light more efficiently than if each molecule acted individually. This effect can also be applied to batteries , since the more molecules with stored energy there are, the more efficiently that energy can be absorbed. Thanks to this, the larger the size of the battery, the faster it will charge.
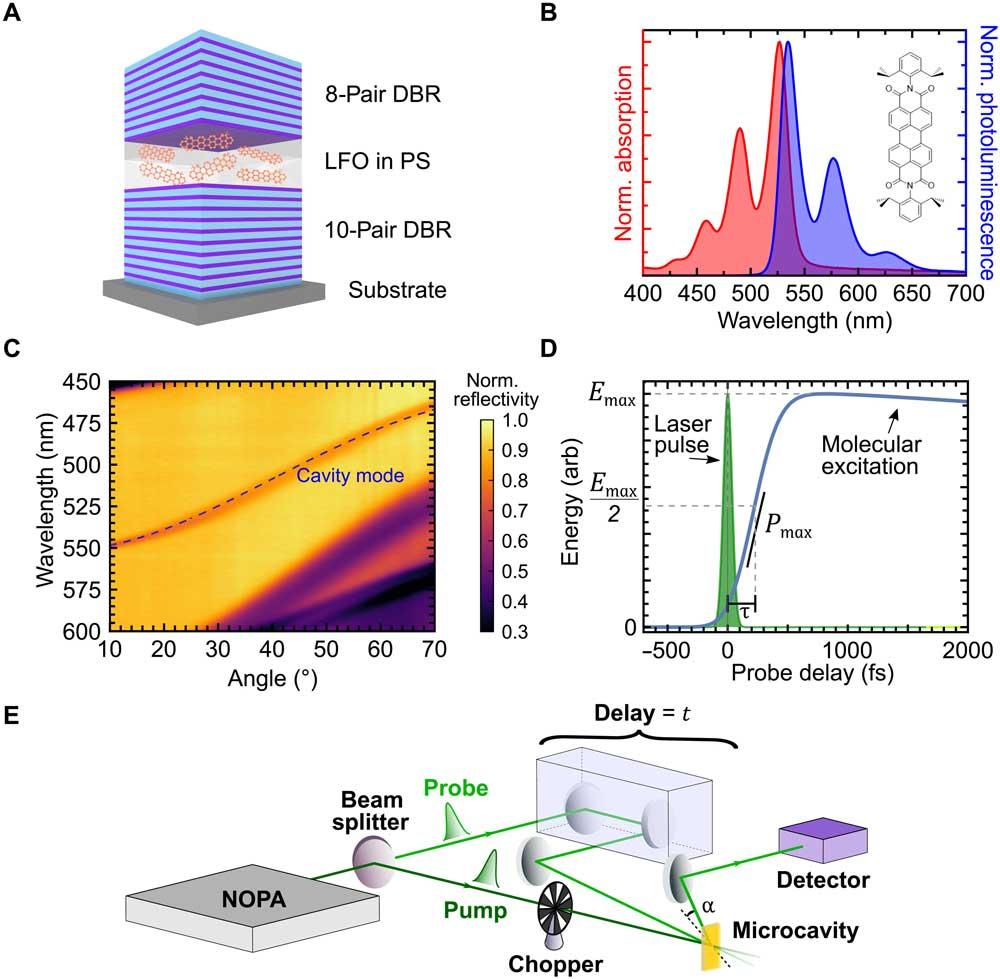
The problem with superabsorption is that it was only a theory, and had not yet been demonstrated on a scale large enough to build quantum batteries. To do this, scientists have placed an active layer of light-absorbing molecules with a dye called Lumogen-F Orange , in a microcavity between two mirrors.
The mirrors were created by using alternating layers of dielectric materials such as silicon dioxide and niobium pentoxide to create what is known as a Distributed Bragg Reflector. The result is mirrors that reflect much more light than a mirror like the one we have at home, which increases the time that the light lasts in the cavity.
Bigger batteries charge faster
They then used ultrafast transient absorption spectroscopy to measure how the dye molecules stored energy and how fast the device discharged. There, they found that as the size of the microcavity and the number of molecules increased , the charging time decreased.
The effects of this research could be very important, since they could allow ultra-fast charging of electric cars and all types of batteries in just a few seconds. The researchers say it’s too early to see what effects these batteries may have, but they are a great first step in bridging the gap between laboratory tests and real-world applications.
Soon, the researchers say they can explore how to combine this system with other ways of storing and transferring energy to create real devices that take advantage of this principle.